
Sample Microgrid Post #1
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Sed malesuada ligula ac augue mattis cursus. Morbi congue accumsan dolor, non ornare neque maximus at. Mauris eu orci sed risus venenatis ultricies. Suspendisse at leo molestie, facilisis sapien in, luctus ligula.
Read More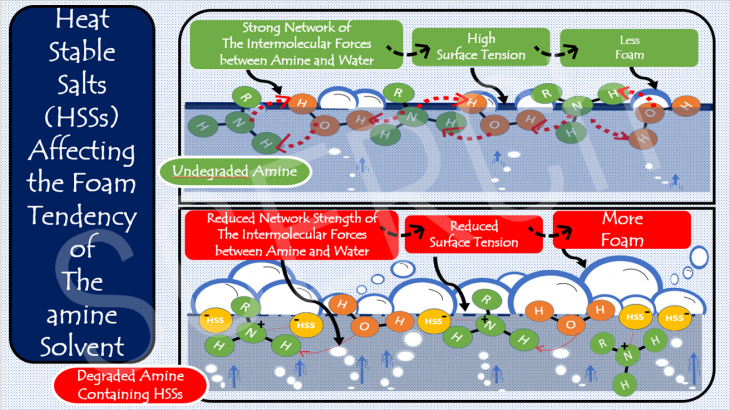
Heat Stable Salts (HSSs) Affecting the Foam Tendancy of the Amine Solvent
The fundamentals of chemistry can help us to understand why heat stable salts (HSSs) make an amine solution more prone to foaming. Foaming is one of the operational issues that can reduce the ability of an amine to capture the CO2.
Read More
Taking Responsibility
Today’s post on Back to Basics is a reminder to all researchers on the importance of TAKING RESPONSIBILITY !!
Read More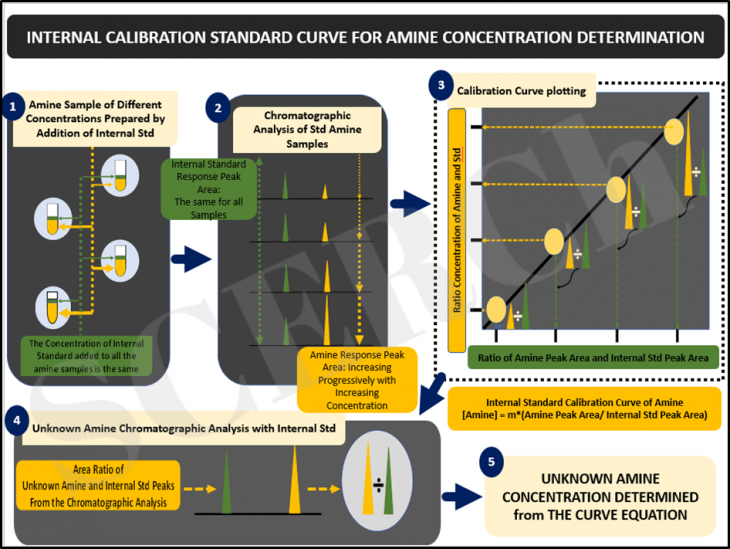
Internal Calibration Standard Curve for Amine Concentration Determination
This post, we explain how the concentration of an unknown amine is determined using the internal standard curve method which is known to help reduce run to run/day to day inconsistency, thus improving the analysis accuracy.
Read More
Determination of Concentration using an External Calibration Curve
If we want to know the concentration of our sample solution, how can we do it?
Read More
What is Filtration?
Filtration is a mechanical or physical operation by which insoluble solid particles are separated from a liquid-solid mixture by forcing the liquid through the pores of a filter...
Read More
Catalyst Washing Processes
Washing is a process that separates soluble impurities from insoluble catalyst particles by dissolving catalysts in a solvent, like water.
Read More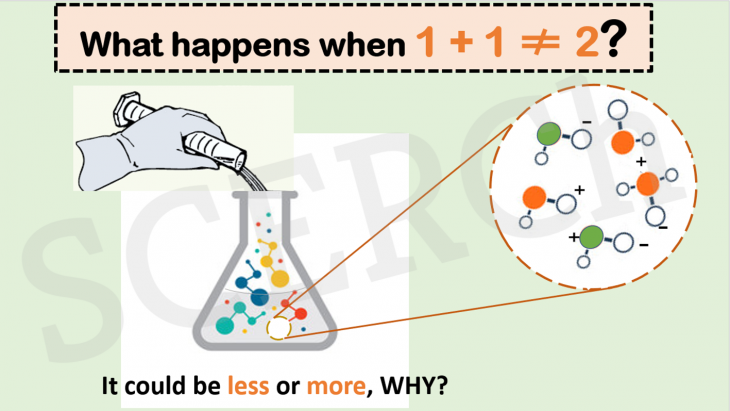
What Happens when 1 + 1 doesn’t equal 2?
In our previous post, we looked at the physical aspect of the volume addition of two different solvents. In this post, we shall review the chemical aspect.
Read More
Did you know that 1 + 1 is not always 2?
When 250 mL of water is added to 250 mL of water or when 250 mL of alcohol is added to 250 mL of alcohol, the final volume will always be 500 mL, as expected. However, when the same water is added to an equal volume of alcohol, the final volume is about 10% less than the original volume of the two liquids. How?
Read More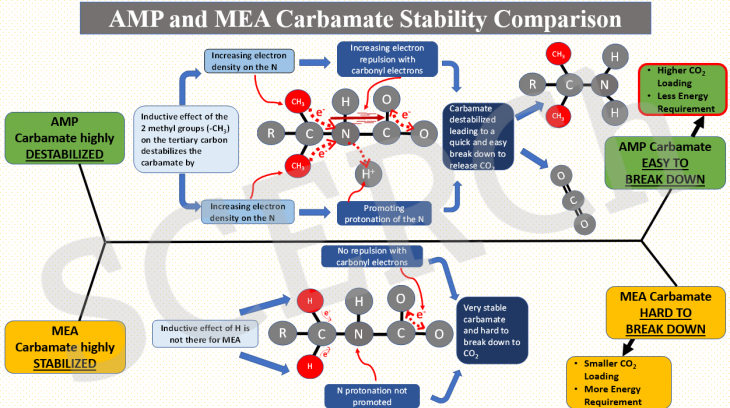
AMP and MEA Carbamate Stability Comparison
In this post, we explain why carbamate of sterically hindered AMP is unstable relative to that of MEA. The instability of AMP carbamate then leads to an easy break down to release CO2 and amine in the desorption process, thus giving rise to a higher CO2 loading and a lower desorption heat requirement than those of MEA.
Read More