Stability Studies of a New Amine Blend for the Capture of CO2
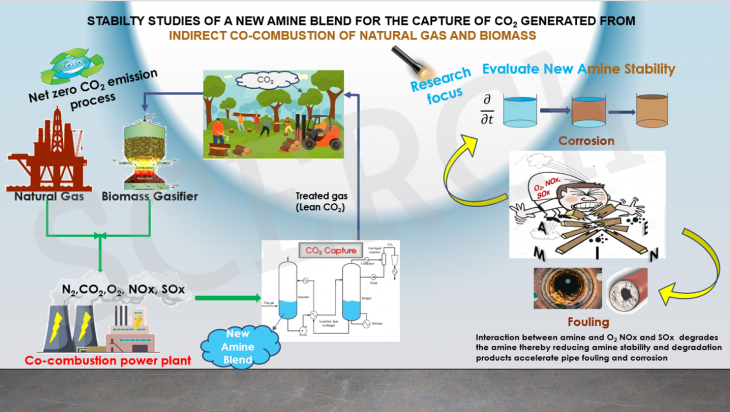
Global warming and climate change due to the excess release of CO2 from human activities has been one of the world’s problems for a number of years now. Many ideologies have been born in the quest to seek a solution to this global problem. One of the captivating solutions is to use right proportions of different sources of fuels together with a post combustion carbon capture technology to achieve net zero or negative CO2 emissions (Avor et al., 2022).
Post combustion carbon capture technology using solvent absorption has proven to be very reliable and efficient. However, some critical challenges faced by investors include solvent cost, solvent loss and cost of solvent regeneration. Monoethanolamine (MEA) being the most explored alkanolamine absorbent and the benchmark for all other absorbents is noted for high absorption rate, low solvent cost and low viscosity. Nevertheless, MEA has high rate of degradation which leads to high solvent loss.
Degradation in the context of CO2 capture process, refers to the loss of active sites of an absorbent. It is caused by the unsought side reaction between an absorbent and either oxygen, NOx, SOx or other flue gas impurities such as particulate matter and heavy metals. High temperatures are also known to aid degrade absorbents to form degradation products. Solvent degradation is an important parameter to consider during solvent selection for commercial use in CO2 capture process. Solvent degradation does not only lead to solvent loss but degradation products like carboxylic acids and heat stable salt (HSS) promotes corrosion. Also, nitrosamines emitted due to some absorbent reaction with NOx poses a threat to human health. There are chances of fouling and solvent foaming due to degradation. Least to mention is the increase in operational cost due to increase in heat duty required for regeneration because of the presence of degradation products.
This work seeks to evaluate the stability of a new amine blend which has better CO2 absorption and cyclic capacity compared to the benchmark, 5M MEA (Avor et., 2022). The degradation studies of the new amine blend will be fully characterised in terms of reaction kinetics and mechanisms. This will be done by running a simulated co-combustion flue gas of biomass and natural gas through the new amine blend at various absorption and desorption conditions for long hours. Also, gases emitted from the study will be analysed to ascertain how safe the process is to our environment.
