AMP and MEA Carbamate Stability Comparison
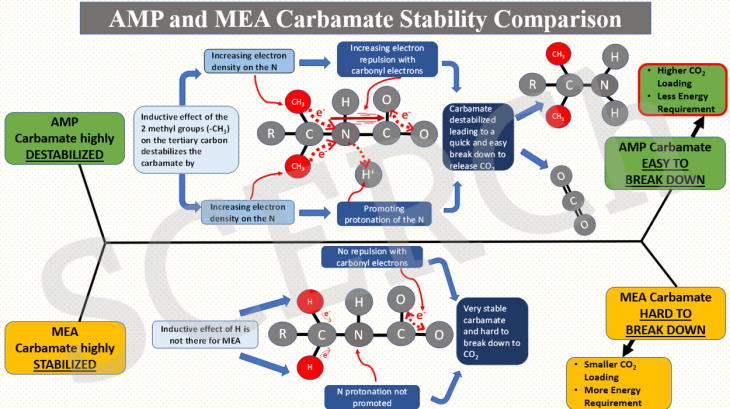
In this post, we explain why carbamate of sterically hindered AMP is unstable relative to that of MEA. The instability of AMP carbamate leads to an easy break down to release CO2 and amine in the desorption process, thus giving rise to a higher CO2 loading and a lower desorption heat requirement than those of MEA. As shown on the graphical abstract, AMP structurally has 2 methyl groups (-CH3) on the tertiary carbon adjacent to the nitrogen while in contrast to MEA, the same carbon has only 2 hydrogens attached to it. Methyl groups in AMP are known as electron releasing groups so that they are relaying their electron density to the tertiary carbon and then the nitrogen.
This phenomenon causes AMP carbamate to destabilize by being explosed to 1) increased electron density on the N which is highly desirable for a reaction with proton from amine (H+) to trigger the carbamate breakdown process and 2) increased electron repulsion with the delocalized electrons on the carbonyl group whose effect destabilizes the carbamate and makes the break-down process more favorable.
In comparison to MEA, the 2 hydrogens (-H) on the tertiary carbon do not have the same inductive effect as seen from the methyl groups. As a result, the carbamate nitrogen reactivity is not enhanced by any extra electron density as seen in AMP carbamate and so its reaction with proton to break down it. The instability effect triggered by electron repulsion with the carbonyl group also is not there. The stability of MEA carbamate is therefore attributed to a combination of these 2 factors which then makes the compound break down process harder to occur compared to that of AMP. The CO2 loading and heat requirement as a result are higher than the case of AMP.
